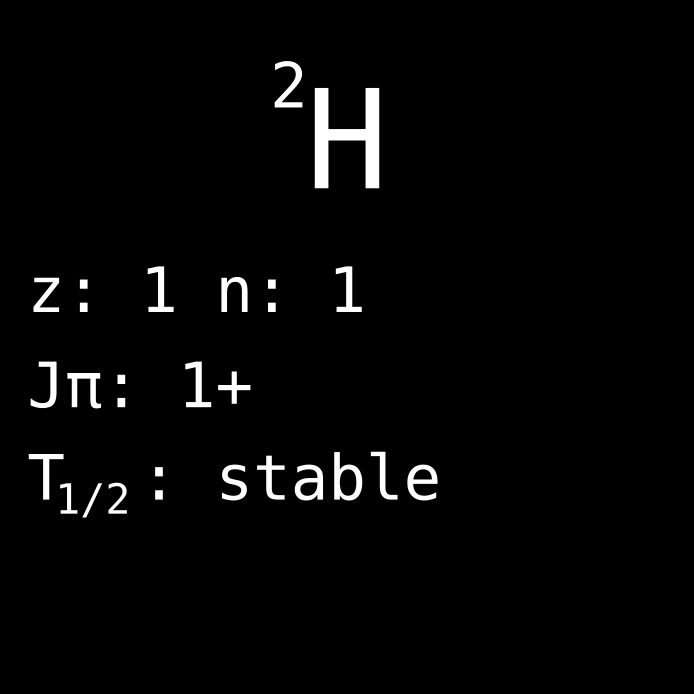

The second isotope of hydrogen has many names, including deuterium and heavy hydrogen. It is the nuclide composed of 1 proton and 1 neutron. Deuterium is stable, and makes up a small proportion of the hydrogen in the world, a little bit more than 1 in 10 000 hydrogen atoms on Earth are deuterium atoms. Deuterium nuclei are the simplest bound system of nucleons considering that neither 2 protons nor 2 neutrons are bound systems. As already mentioned December 1st, deuterium has also an important role in the burning of hydrogen in the sun.
But the more interesting thing about deuterium is its technological usefulness. Despite being a costly process, isotopic enrichment is being used to produce deuterium. To understand why we do this, we need to remember something about uranium-235 that was mentioned a few days ago, the uranium is more reactive to fission if the neutrons are slowed down. In fission the neutrons are released at great velocities, and the only way to slow them down is by letting them collide against materials in the vicinity.
The materials that are good at slowing down the neutrons are called moderators. There are two properties that make a good moderator, it should be light, and it must also not be prone to absorb neutrons. The light hydrogen is of course the very lightest of all nuclides, and weighs almost as much as a neutron. This means that a neutron can lose all of its speed in a single collision with hydrogen, like a billiard ball when hitting another billiard ball head on, it will come to a complete stop. Compare that with the nuclear fuel itself, consisting of uranium with more than 200 times heavier nuclei than the neutron. This is like bouncing a ping pong ball against a bowling ball. The lighter ball will lose almost none of its energy in a collision, and the heavy ball will remain at rest. So we need to put a moderator (with some billiard balls) in the spaces between the nuclear fuels (the bowling balls).
Considering this, the light hydrogen may actually seem like the best option. However, some neutrons are lost because of being captured by the light hydrogen, and lost neutrons will of course not cause any fission reactions. The deuterium absorbs nearly no neutrons. All in all, this makes heavy water the best moderator material.
With heavy water for moderation, a nuclear reactor can reach criticality (meaning to achieve a self-sustained chain reaction) even with natural uranium, which has only 0.7% fissile uranium-235. Therefore it was used a lot in reactors of the past, and it still is used, but in a minority of existing reactors. Our most common commercial reactors today actually make use of light water, but this is only possible because nowadays we have high-capacity uranium enrichment facilities, where the proportion of the fissile U-235 can be increased from its very low natural concentration.
Deuterium had a famous role to play in the second world war. The Germans used a factory in Lillehammer, of occupied Norway, to produce heavy water moderator for their nuclear weapon development. This factory was sabotaged by the Allies, thereby slowing down any German attempts to obtain the nuclear bomb.
Apart from current use in some reactors, and it’s place in history, it must also be mentioned that it has a place in the future. In thermonuclear fusion, small nuclides are knocked together into heavier, and in this process some mass disappears, which is released as heat. Unlike the thermonuclear fusion in the sun, protons are not suitable for fusion on Earth. While there are a few different fusion reactions that are being considered, deuterium is involved in most of the interesting ones. Therefore, we may in the end rely on nuclear energy, where the deuterium is not merely acting as a moderator, but is a primary energy source that is practically inexhaustible.
Finally, according to hearsay, there was once a Norwegian bar where drinks were served with ice cubes by heavy water. The slightly heavier ice of heavy water sinks to the bottom of the drink, which can seem a bit spectacular. Isn’t that a cool application of the second lightest nuclide in our universe?

© 2020 Zs. Elter, P. Andersson and A. Al-Adili