
Cesium-137 with its 55 protons and 82 neutrons has a bit of a bad reputation. Cs-137 is a major fission product produced in more than 6% of the fissions that happen in nuclear reactors. It’s also a strong emitter of gamma radiation, emitting a 662 kiloelectronVolt (keV) gamma-ray (technically, it should be mentioned that the gamma-ray is emitted by the short-lived offspring, Ba-137m.) In addition, the half-life of the cesium isotope is 30 years. This means that if released in a reactor accident, it leaves a long-lasting radioactive contamination in the environment. In addition, cesium is a volatile element, which all in all makes it a nuclide of particular concern in reactor accidents. In both the Fukushima and Chernobyl accidents, this nuclide has been the main concern in the affected areas.
However, beside this negative aspect, it is also a nuclide that has found use in lots of applications, much because of the same reasons, being such a strong and long-lasting gamma emitter. During decades after removing a spent nuclear fuel from a reactor core, the 662 keV gamma-ray from Cs-137 is a dominating part of its radiation. This has come to be commonly used in order to inspect fuels. This way, one can confirm the presence of the irradiated fuel non-invasively, something that is in the interest of the International Atomic Energy Agency, and is used by their inspectors that are sent to countries that have signed the treaty on non-proliferation of nuclear weapons (NPT). It is also used to study the nuclear burnup of the fuel (ie. how much energy was extracted as heat from the fuel during operation), to confirm that the power in the reactor has been distributed the same way as predicted by the reactor operators. Another interesting application is performing tomography on fuel elements by measuring Cs-137 from different detector positions around the fuel. This works pretty much like a SPECT scan in medicine, and the reconstructed emission intensity of Cs-137 can show the burnup within the fuel element.
Beside the direct use of Cs-137 in the spent fuel, it is also chemically separated in order to create gamma sources for scientific and industrial use. In medicine, sealed sources of Cs-137 are used for radiation cancer therapy, and as a radioactive tracer. Food irradiation is another field of use, where the radiation from a sealed source is used to kill pathogens in food, such as salmonella and E.coli bacteria, which are responsible for millions of infections every year, and a large number of deaths.
The nuclear weapons tests performed during the 1950-ies and 1960-ies caused releases of Cs-137 to the atmosphere. Because of this, trace levels of Cs-137 are now present everywhere. Although the activity concentrations are very small, well below natural radioactivity levels, it can still be measured with sensitive instruments. This has been used to verify the authenticity of vintage wine bottles. Wines produced before the nuclear weapons tests and stored hermetically, should not contain any Cs-137, if authentic.
Due to the same reasons, in sensitive radiation measurement setups we prefer to use so-called low background steels to shield the experiment. Such steels are ones which were produced before the nuclear weapons tests (for example by retrieving steels from sunken ships), therefore they do not contain any trace of cesium that could interfere with measurements.
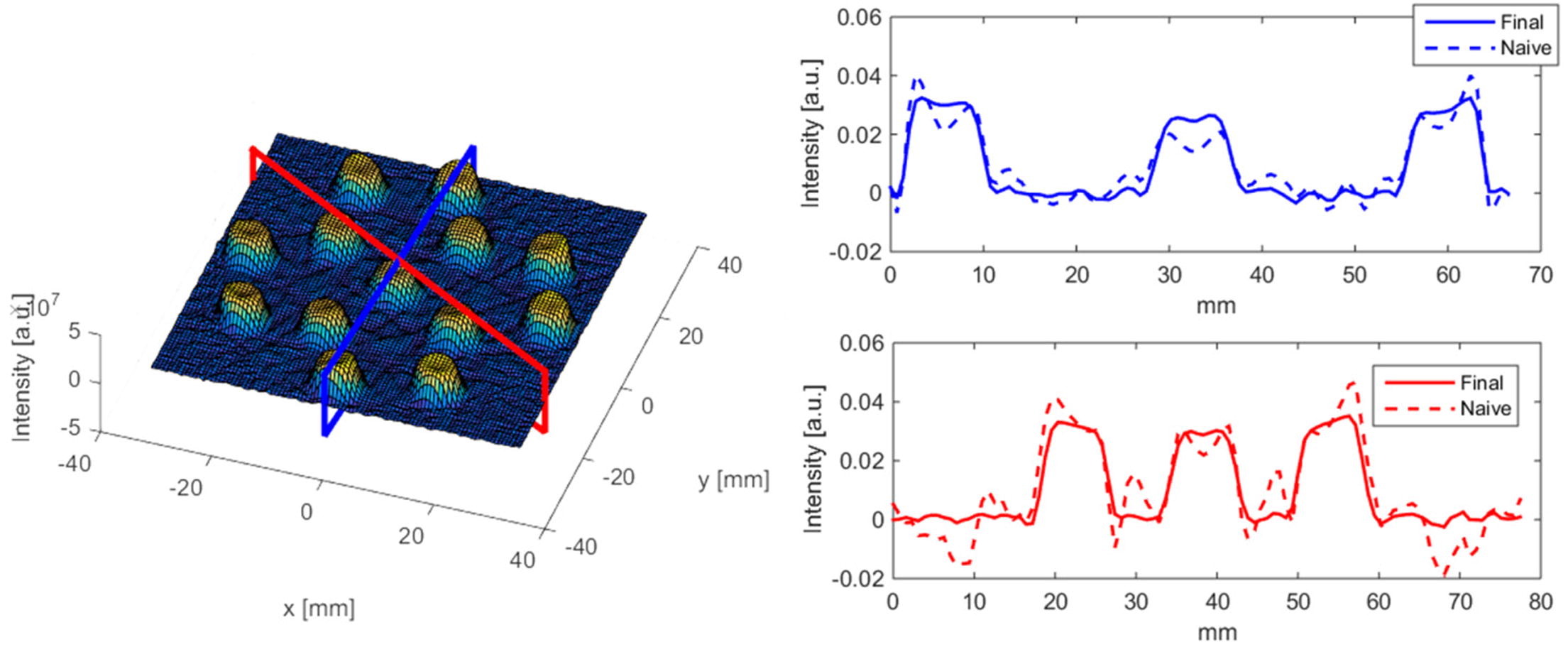
Tomography reconstruction of Cs-137 measured in an assembly of 13 fuel rods. (Image with permission from: doi.org/10.1016/j.anucene.2017.06.025)
© 2020 Zs. Elter, P. Andersson and A. Al-Adili