

Radon-222 (86 protons, 136 neutrons) is a radioactive nuclide that occurs naturally on Earth. It’s half-life is merely 3.8 days, and it decays by alpha decay, sending out a He-4 nucleus. We planned to introduce the favorite nuclides of our colleagues and friends, but apparently Rn-222 is not a likeable nuclide, since nobody proposed it. As we will explain later, radon is one of the main causes for lung cancer, it is understandable that no one likes it. However, since it has significant implications on our life and health, we have decided to include it in this calendar.
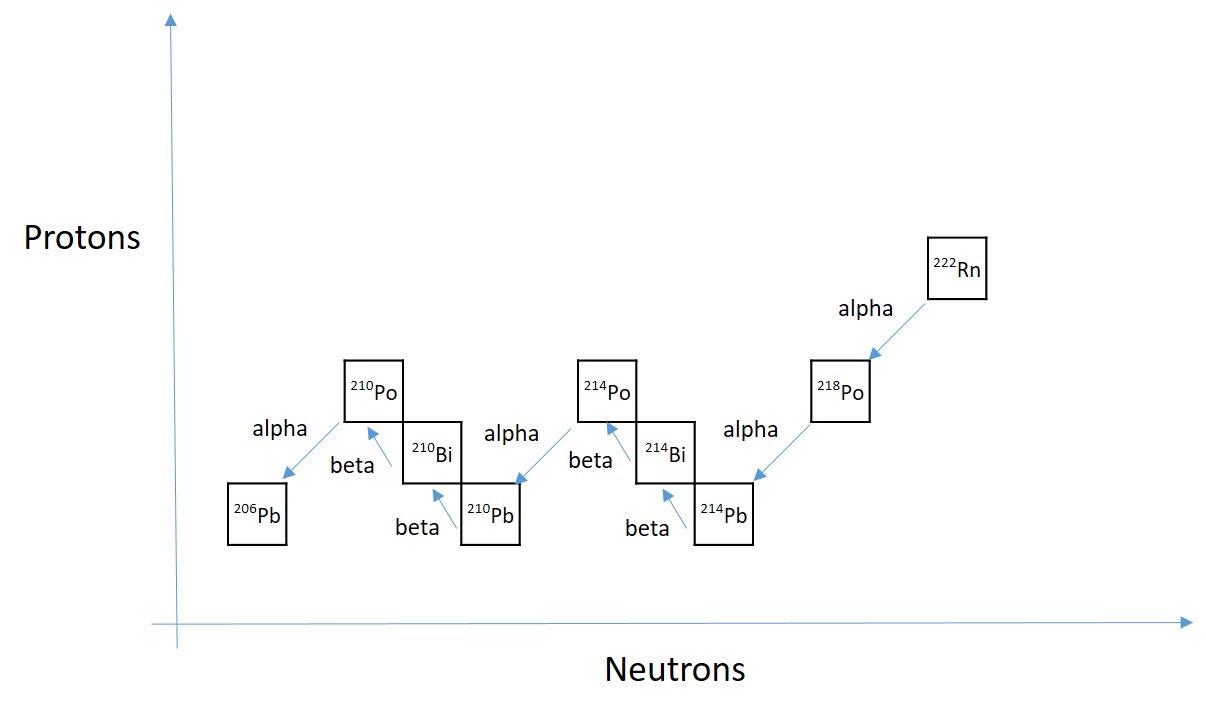
The reason that Rn-222 is abundant on Earth, despite its short half-life, is that it is a part of the uranium series. That is the series of radioactive decays that follow the decay of long lived actinide uranium-238. Ra-222 is the seventh nuclide in that sequence, the closest precursor is radium-226. With a decay series starting from a very long-lived parent, such as U-238 (half-life of 4.5 billion years), the activity of all the children (meaning the rate of decay) is equal to the activity of the parent nuclide. This is called secular equilibrium.
The claim to fame of Rn-222, is that it is the cause of most of the radiation dose exposure to the public. Radon is formed naturally in the ground, and the 3.8 days half-life is just enough that it may escape to the atmosphere, as it is chemically a noble gas, it will not participate in any chemical reactions bounding it to the soil. This is normally not a problem, but can be a serious health hazard in old houses with cracks in the foundation in areas where uranium concentration is high in the ground, the air is still, or in buildings that are made of material with high level of the precursor nuclides, as well as in caves or mines. If you live in a cave, it is a good idea to move to a house and if you live in an old house, it is a good idea to open your windows sometimes.
Alpha radiation such as from radon has a short range in matter, and is even stopped effectively by the outer layers of skin. Thus, external alpha radiation does not pose a health risk. However, internal exposure, when the radioactive alpha source is inside our body (for example during inhalation of radon), the energetic alphas have direct access to sensitive tissue for example lung tissues. And that’s just the beginning, because the decay series continues, and now the source has been immobilized in the lungs, where it is the most harmful. A large number of decays follow, before the final destination of the uranium decay series is reached, which is stable lead-206. The first five decays happen in a very quick succession and end at lead-210. This isotope delays further progression with its half-life of 22 years.
© 2020 Zs. Elter, P. Andersson and A. Al-Adili