

The nucleons (protons and neutrons) in a nucleus all interact with each other: neutrons with neutrons and protons through the strong nuclear force, protons with protons through the electromagnetic and strong nuclear force. These forces have different strength and range therefore the nucleus is a complex multibody system. Due to this, certain nucleons sometimes can build clusters within the nucleus. One such example are the Borromean nuclei. A Borromean nucleus is an atomic nucleus comprising three bound components in which any subsystem of two components is unbound.
Helium-6 is a borromean nuclide, believed to consist of a helium-4 nucleus surrounded by a halo of two neutrons. While helium-6 is unstable with a half-life of less than a second, it is notable that a single neutron would not be bound at all to the helium-4, and neither are two neutrons bound to each other. It is only in the presence of the 3 systems together that they stick, at least for some time until converting by beta decay to a lithium-6 nucleus. There are only a handful of nuclei which are borromean and He-6 is the simplest of them.
What is the difference between “unbound” and “unstable”? Well we get examples of both in the helium isotopes. Starting with two protons and two neutrons in a helium-4 system, that is very stable. A lot of binding energy is released once the 4 free nucleons join up in the He-4. Making the He-4 is like letting rocks fall down in a well, they won’t make any resistance. But add another neutron to make He-5, and the binding energy of the system actually decreases. This means that the system will spit out that neutron as soon as it can. Putting this fifth neutron in there, is like throwing a rock up in the air. Although it is possible to do it, it will surely come right back again. That’s an unbound neutron. But if yet another neutron is added so we get our nuclide of the day, He-6, then the binding energy is increasing again, making every nucleon bound. All the rocks are in the well again, happily ever… well not forever. He-6 is still unstable, because one neutron can beta decay to a proton, changing the nuclide from He-6 to Li-6 (Lithium). However, this is a slow process that could take about a second. Compare that with the decay of the unbound neutron in He-5 that would happen in about 0.000000000000000000001 second.
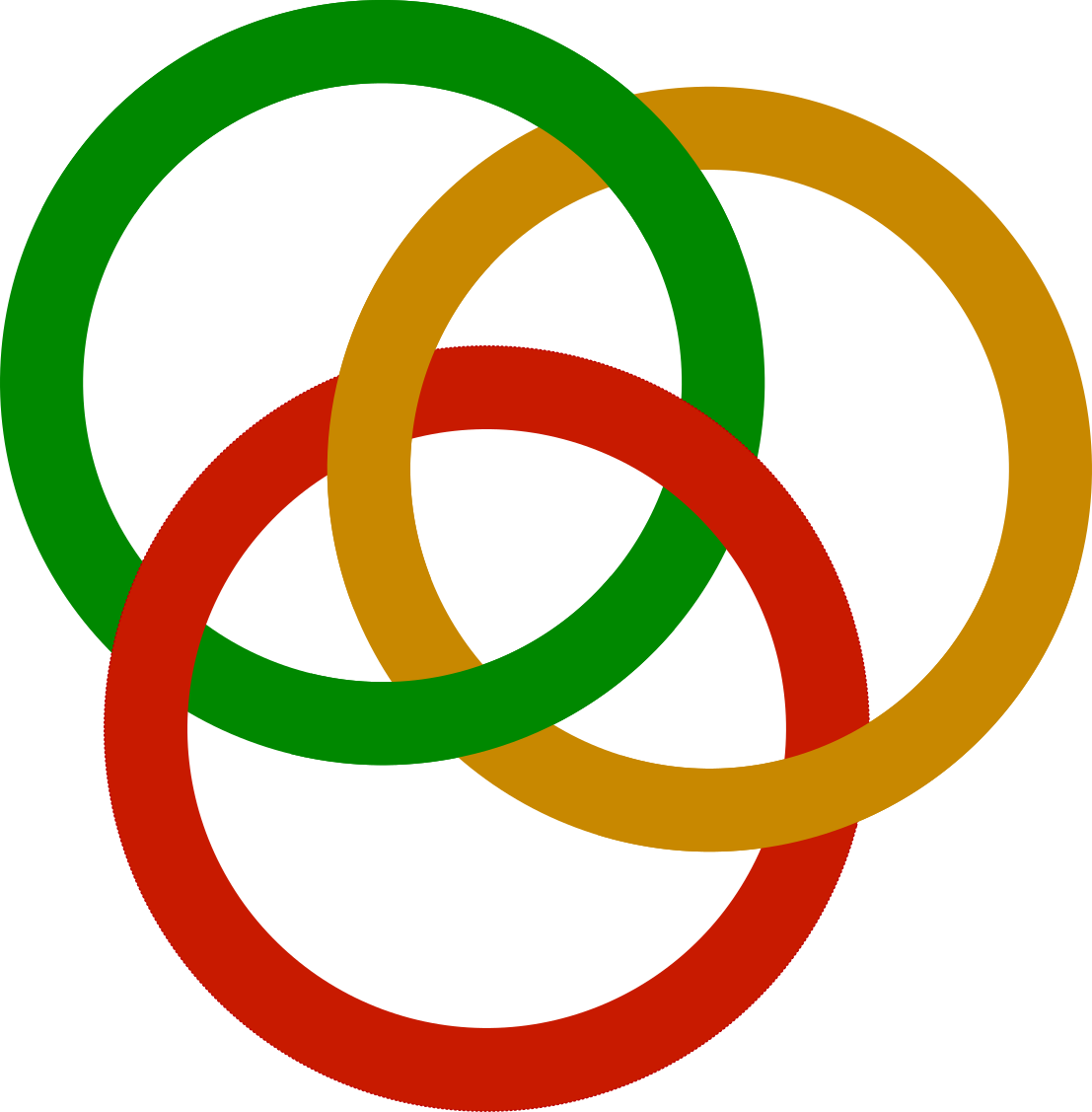
By the way, the name Borromean is inspired by the intertwined rings on the coat of arms of the Borromeo family from Italy. Just as the He-6, no two rings are bound unless the third ring is present.
© 2020 Zs. Elter, P. Andersson and A. Al-Adili