

Technetium-99m with 43 protons and 56 neutrons does not exist in nature. Neither does any of its isotopic siblings. Ever since Mendelejev’s invention of the periodic system it was clear that a gap was present at the atom number 43. Despite searches for this element, it couldn’t be found. The reason for this gap is that all nuclides with 43 protons are radioactive. The longest living isotopes, with mass numbers 97 respectively 98, both have half-lives of about 4 million years. While this is relatively long-lived, it is still much shorter than the age of our planet, so therefore nothing remains present on Earth. It was only with the invention of particle accelerators that the element could be artificially produced in observable quantities. The element was given the name technetium, which reflects that artificial origin.
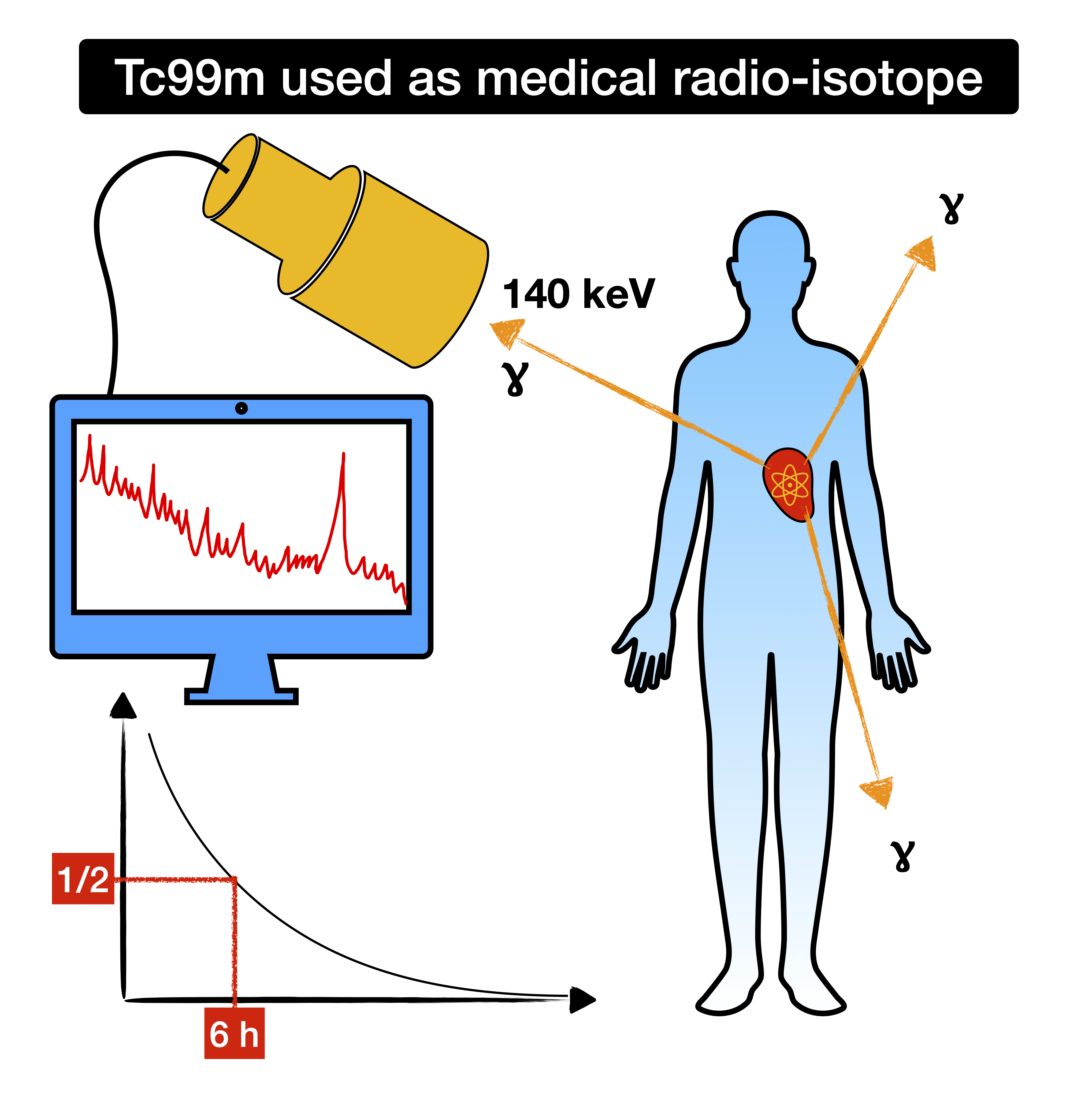
Today, technetium has a very important role in nuclear medicine. In particular, the radioactive Tc-99m is used as a gamma emitting tracer in various diagnostic tests. There are many properties of Tc-99m that makes it particularly suitable for use in medicine. Among these are:
Because of these favourable properties, Tc-99m is produced in nuclear reactors and it is used in millions of diagnostic procedures every year, like a workhorse in nuclear medicine, used to diagnose many different injuries, infections and abnormalities, often with greek or latin names.
There are however only a few nuclear reactors in the world that are producing technetium, and that has occasionally caused shortages in recent years. Still, even when not a shortage, one may wonder how the nuclide can be transported fast enough from the production reactor to the medical facilities somewhere in the world, where it is needed. For this purpose, there’s actually another nuclide that’s produced in the reactor, radioactive molybdenum-99 (Mo-99). This is produced by neutron irradiation of uranium, and as a result, molybdenum-99 as well as many other nuclides are produced. After chemical separation of the molybdenum, it can be transported rapidly across the world. Mo-99 has a half-life of a few days, so it will produce our nuclide, Tc-99m, by radioactive decays, which can then be separated and used for medical diagnostics. Despite the short half-life of Tc-99m, the continuous replacement makes it possible to milk it for about a week.
Maybe you are wondering about the letter ‘m’ in the nuclide’s name? It indicates that the nuclide is a metastable isomer of Tc99. This means that the configuration is an excited state of the isotope that is relatively stable (it exists for hours after all), despite not being the ground state (lowest energy state) of the particular isotope. Meta-stable states have difficulty decaying to the ground state due to a large difference in their nuclear spin. What’s spin you said? That’s angular momentum, glad to help.
© 2020 Zs. Elter, P. Andersson and A. Al-Adili