

Carbon-14 having 6 protons and 8 neutrons is the foundation of a forever young pun between scientists, the only type of humour a scientist can really appreciate. The reason is that the main application of C-14 is in carbon dating. Can you guess the joke?
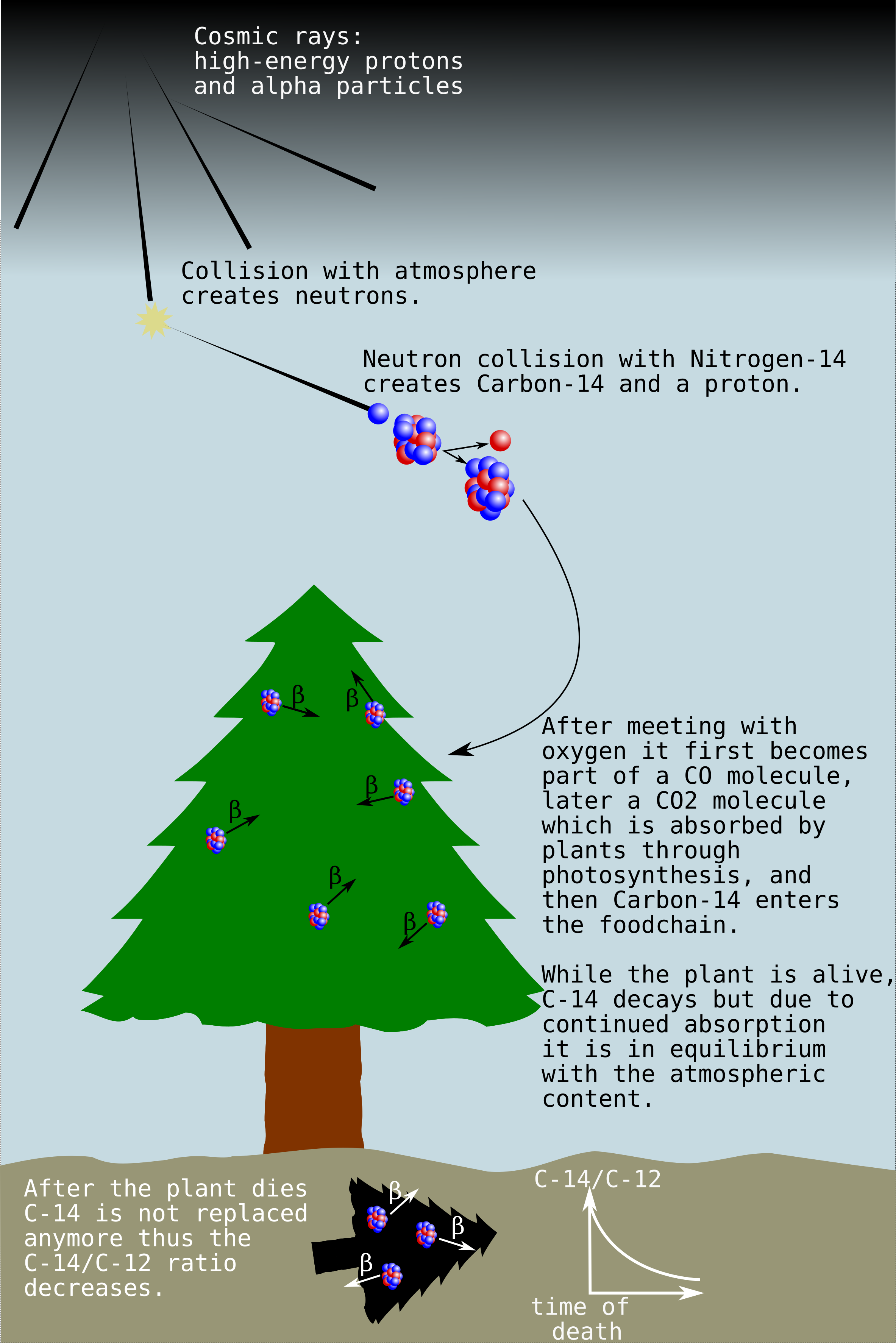
C-14 is a radioactive isotope, with a half-life of 5730 years. C-14 is rare (one in a trillion carbon atoms), but occurs naturally. It is a cosmogenic nuclide, continuously created due to the interaction of cosmic rays on nitrogen nuclides in the atmosphere as explained on the image below. Therefore, any living creature (animal or plant) is going to take up C-14 besides other stable carbon isotopes from air. However once they die, the radiocarbon continues to decay in their organic remains without being replaced by newly absorbed radiocarbon, thus the ratio of C-14 to the stable C-12 isotope is going to change. In case one measures this ratio, and compares it to the ratio observed in the atmosphere, it is possible to deduce how long ago the plant or animal died. This is called carbon dating. Unfortunately the half-life of C-14 poses a limit to how far in the past we can trace, since once there is too little C-14 left in a sample, roughly after 60000 years, it is not possible to estimate the age of it. Luckily, 60000 years is already a lot for archaeology.
It is worth mentioning that since humans made atmospheric nuclear weapons tests in the 50-ies and 60-ies, the ratio of C-14 was raised a little during that time. On the contrary, because of the increasing burning of fossil fuels (coal, oil and gas) that are millions of years old (and already depleted of C-14), we are currently pushing out a lot of CO2 without C-14 into the atmosphere, thus our great species has managed to alter the radiocarbon ratio, meaning that future archaeologist will need to recalibrate their carbon dating measurements and take into account these effects.
Finally the joke: “Are you C-14, because I would like to date you?”
© 2020 Zs. Elter, P. Andersson and A. Al-Adili