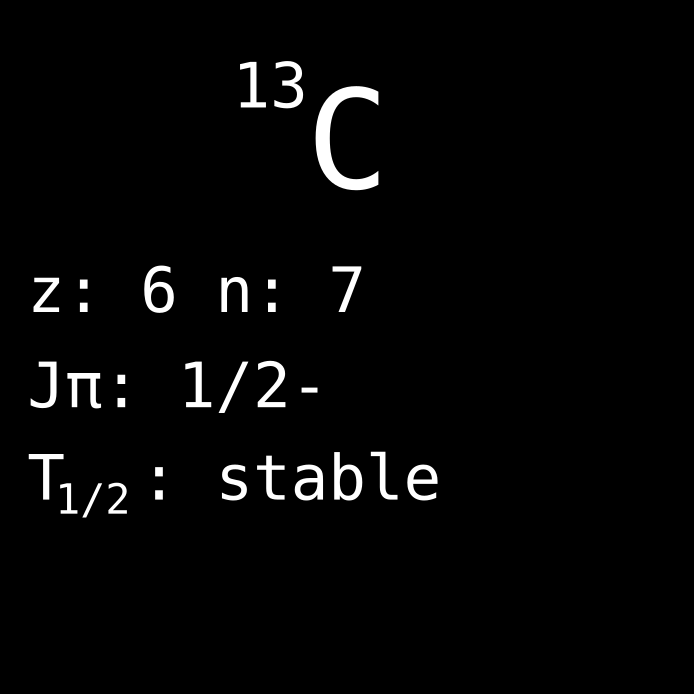

Each nuclide has certain properties. Some of these properties are fairly familiar also for the unintroduced, such as the mass and the electric charge. In addition, the nucleus has a more esoteric property, the spin. The spin is derived from the building blocks of the nucleus, meaning its protons and neutrons. These are so-called fermions, which are magnetic dipoles, such as if being a spinning electric charge. This is quantified by their spin number that is a half, 1/2, for both types of nucleons. In nuclei that have an even number of both protons and neutrons, they pair up, with one spin in each direction. Therefore, their spins cancel out, and these nuclei thus have 0 spin. But today’s nuclide, carbon-13, has an unpaired neutron, so it has a non-zero spin. This turns out to be important.
In Nuclear Magnetic Resonance spectroscopy (NMR), oscillating external magnetic fields are used to interact with the nuclear spin, to flip the nuclides over. However, the molecular structure also affects the interaction of the external magnets with the nuclear spins, and this is used to retrieve information about the compounds in the inspected object. NMR can be highly useful for analysis of various kinds of mixtures. In addition, a similar technique is used in medical imaging, then called Magnetic Resonance Imaging (MRI), which is for many different diagnostic purposes. MRI has the advantage over traditional X-ray radiography that no ionizing radiation is used.
For an element to be studied using NMR, however, its isotopes need to have non-zero spin, since only they can be assessed this way. If the nucleus is not magnetic, you can oscillate the magnetic field as much as you want, the nuclei won’t flip. This presents a challenge with regards to carbon, which is a very important element in many biological processes, since the most naturally abundant isotope, carbon-12, unfortunately has zero spin. However, as said our nuclide of the day, carbon-13 has an unmatched neutron, causing a non-zero spin of the nucleus. Because of this, the less abundant isotope carbon-13 is of particular importance for use in NMR. Pure carbon-13 is continuously produced by isotopic enrichment of natural carbon, for use in NMR spectroscopy.

© 2020 Zs. Elter, P. Andersson and A. Al-Adili