
There are no naturally abundant elements (on Earth) with atomic number greater than 92 (with the highest being uranium). Heavier elements have been around in the young Earth, or in the interstellar dust from which our solar system was created. However, they were too short-lived to remain in significant quantities today.
Heavier elements than uranium are only present due to artificial production. They are produced in substantial amounts by the neutron irradiation used in the nuclear fuel cycles. In this manner, heavier elements are gradually produced by consecutive neutron capture reactions, starting from the uranium-238 present in the nuclear fuel.
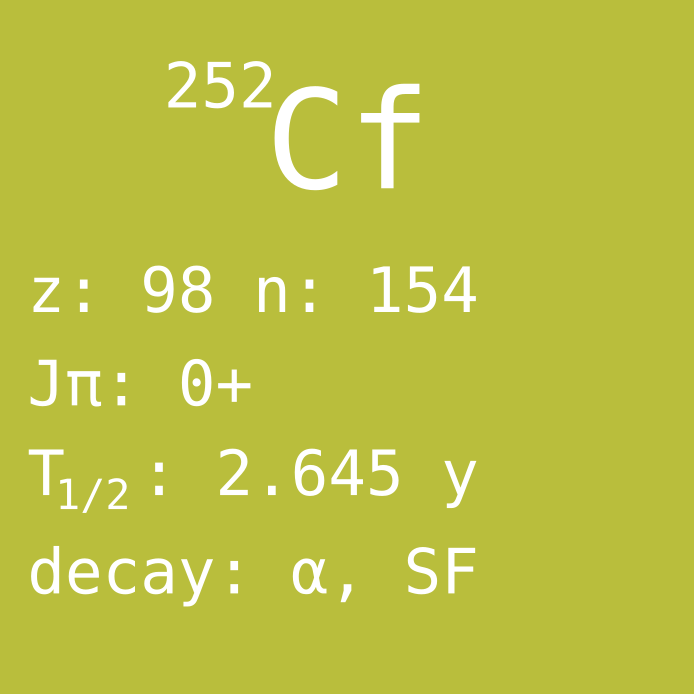
Californium is an atomic nucleus with 98 protons and was named after the U.S. state of California after the discovery at University of California Radiation Laboratory. One particular isotope is californium-252, which has 6 protons more than natural uranium, and it has in total 14 more nucleons (considering both the protons and neutrons). Consequently, the original uranium atom must have captured 14 neutrons, and also undergone 6 beta decays, in order to create a californium-252 nucleus. In addition, many atoms would be lost on the way, due to induced fission reactions that are also caused by the neutron absorption, or due to undergoing the wrong type of decay somewhere on the way. All in all, thousands of neutron absorptions are needed for every Cf-252 nucleus that is produced. It is a challenge to expose the original material for so much neutron fluence for so little californium produced. Therefore, it is impressive that any amounts of californium can be manufactured at all, and that it exists on the market. Cf-252 is one of the most expensive substances in the world (easily beating diamond or gold).
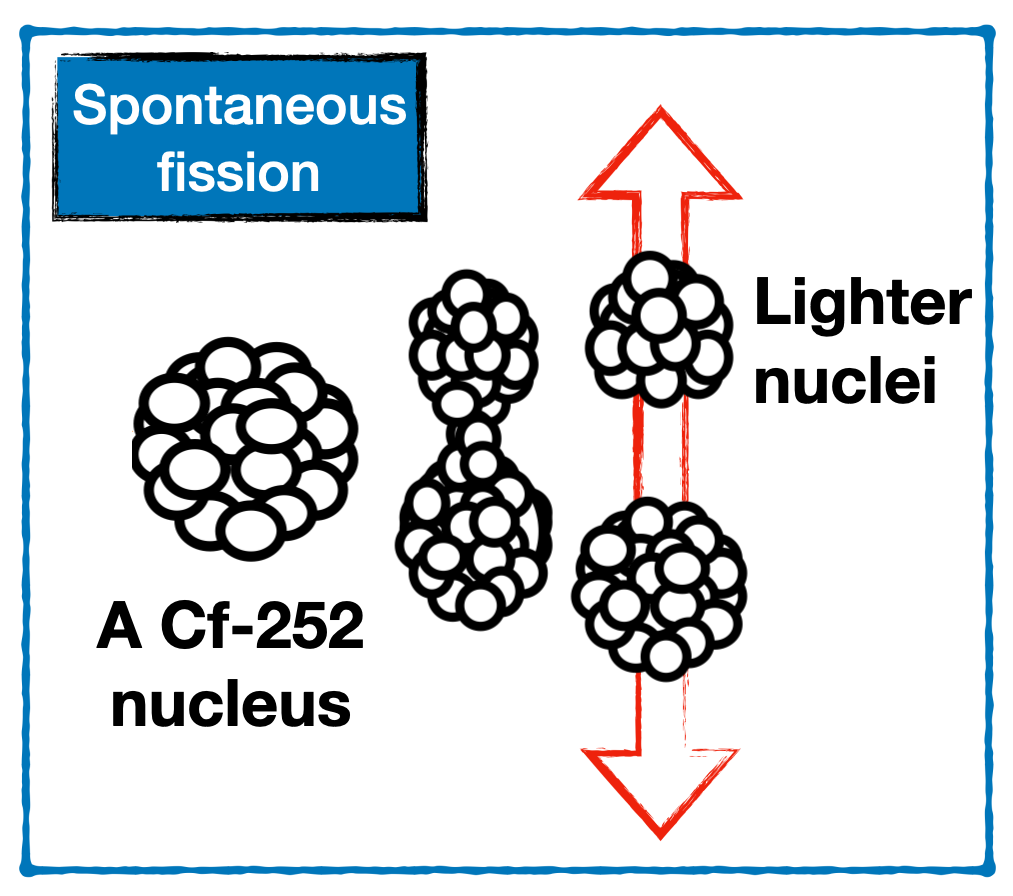
The reason why Cf-252 is worth all this effort to produce, is the unusual decay properties. It decays by alpha decay (97%) and by spontaneous fission (3%). Spontaneous fission is a spectacular phenomenon, when a heavy nucleus suddenly decides to split, without influence of any incoming particle or energy! In this process, on average 3.8 neutrons are emitted from the lighter fission fragments. A single microgram of Cf-252 produces millions of neutrons every second. Such a strong source of neutron has both scientific and industrial uses. One example is the use in nuclear reactors, where it can be used to start the nuclear chain reaction by feeding neutrons to the core.
© 2020 Zs. Elter, P. Andersson and A. Al-Adili