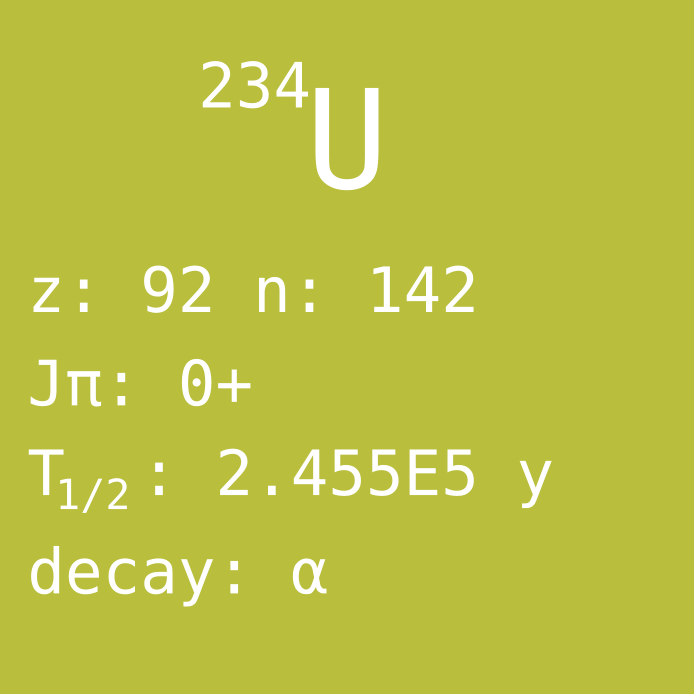

U-234 is the third naturally existing uranium isotope, but at a very small abundance of 0.0055%. U-234 has too short half-life (250 000 years) to be remaining in the Earth since the planet formed. However, the nuclide is a part of the natural decay series, as it is produced mainly by alpha decay of U-238 followed by two beta decays. This results in such a small abundance that it is often forgotten when talking about the isotopic content of natural uranium.
Because U-234 is relatively long lived (it is short lived compared to U-235 and U-238, or compared to the age of Earth, but it’s half life is still quite long), the nuclide is used for uranium-uranium dating. If a forming rock takes up uranium from the environment, then in the first time the isotopes may not be at equilibrium. But one may ask the question: If the isotopes are in equilibrium in nature, why would they not be in the forming rock? One explanation is that the uranium-234 has formed by decays of the uranium-238, causing a kick-back of the nucleus, mobilizing the atom. For example uranium in sea water has a larger proportion of uranium-234. During the first hundreds of thousands of years passing after the formation of new rock material, the U-234/U-238 ratio is thus useful for dating it. However, for this to be possible, the object to be dated must also have formed in a way that made it take up uranium of a known isotopic ratio from the environment. This can be the case in for example stalagmites and corals.
In addition to natural abundance, small amounts of U-234 can be produced by milking it from Plutonium-238 (presented a few days ago), which decays by alpha emission directly to U-234. Meaning that for example the Mars rover Curiosity will eventually be a junk rover with a big chunk of uranium-234 in its dead power source. While the U-234 might be valuable because of its scarcity, it will not produce sufficient decay heat for continued operation of the vehicle. But aliens that come to visit it will be confused if they use uranium-uranium dating, because there will be no uranium-238.
Physics-wise, U-234 is fascinating! Technologically, U-235 always outshines its younger brother U-234, since it is the fissile fuel used in nuclear reactors and therefore is much better studied. Also, uranium-233, which does not even occur in nature, gets a place in the spotlight, due to its role in the thorium nuclear fuel cycle. Still, the lesser known U-234 reveals some exciting physical properties, owing to its even-even nature. When U-234 absorbs a neutron, it becomes odd-even (neutron and proton number), which means it is a threshold reaction. It is not prone to fission with thermal (slow) neutrons, but it is fissionable with higher neutron energies, then the neutron brings the energy needed to split the nucleus in two.
By varying the kinetic energy of the neutron hitting the U-234 nucleus, one can study the characteristics of its fission reaction. We then observe resonance structures which affect the fission dynamics, such as kinetic energy of fission fragments, and their mass- and angular distributions. This is highly interesting for the nuclear physics behind fission and in particular how resonances behave. When U-235 absorbs a neutron it becomes too highly excited and similar subtle effects are not visible anymore.
Even though U-235 is very important for applications, and rightly so, U-234 can sit there and smile for its exciting mysteries. It is selective and just chooses to reveal its secrets to the very few scientists who investigate it in the lab! Don’t worry U-234, we still love U!

© 2020 Zs. Elter, P. Andersson and A. Al-Adili