

Gold-197. People would have killed for it for centuries. Alchemists tried to produce it from other metals. Pirates hid it on uninhabited islands. We put it on the finger of our loved ones. It is just so shiny that it would be impossible not to fall in love with it. Unfortunately we do not have a large amount of it, around 200,000 tonnes above ground, and half of that around the neck of Mr. T.
Gold as an element is one of the least reactive, it is resistant to most of the acids and resists corrosion. But this has not much to do with the nuclear properties of the nuclide, rather with the chemical properties of the gold atom.
From a nuclear physics point of view its most interesting characteristic is being the heaviest monoisotopic stable element, which means that it has only one stable isotope, all the other 36 isotopes of gold are radioactive.
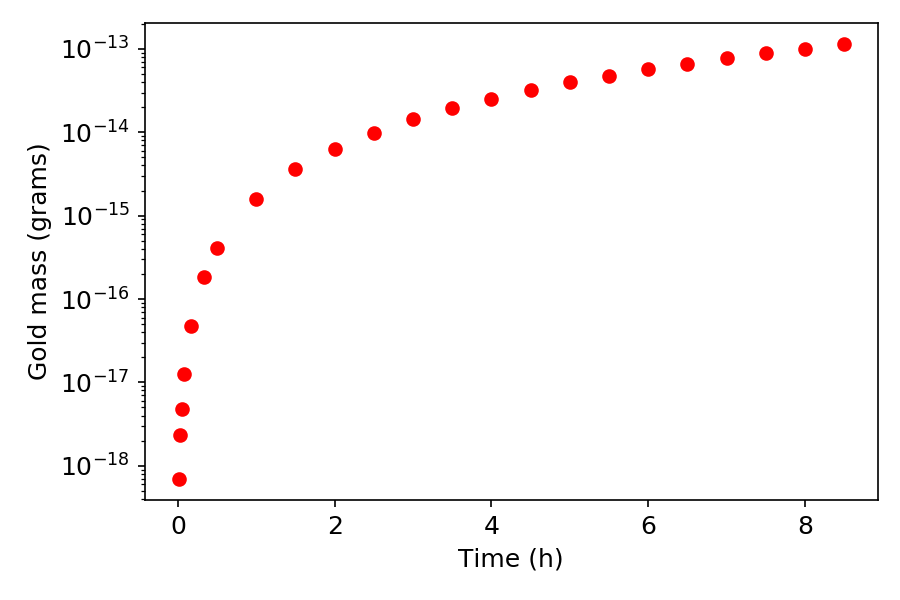
Nevertheless, in the age of Nuclear Physics scientists managed to reach the ancient dream of alchemy, in 1924 Hantaro Nagaoka managed to synthesize gold by bombarding mercury with neutrons. The same can be done in nuclear reactors or particle accelerators. The figure below shows how much gold we would produce in our future neutron facility NESSA at Uppsala University by irradiating a 1 kilo target of Mercury for 8 hours. As we see, this way we would produce picograms, which costs much less then running the generator for 8 hours (and then one still needs to consider how to separate that tiny amount of gold from the target material). One reason for the low efficiency of the production is the above mentioned fact: there is only one stable isotope of gold, so in order to produce usable gold we need to produce exactly that one, which limits the possible pathways leading to gold.
The two best pathways are Hg198(n,2n) (meaning that a neutron enters the mercury-198 isotope, and two neutrons are knocked out), or the neutron capture on mercury-196, the Hg196(n,g) reaction (meaning that a neutron is captured and a gamma ray is leaving the nucleus). Both produce mercury-197, which then beta decays to our precious Au-197. However, the second reaction is most useful with low energy neutrons, whereas we will produce high energy neutrons in our lab, so we are stuck with the less efficient first reaction. With a light water nuclear reactor one has a higher neutron flux of low energy neutrons, and could therefore produce a bit more gold. Some care would also be needed to let the gold activation decay away (some gold-197 would capture additional neutrons, becoming radioactive gold-198, which will decay away in a few weeks with its half-life of about 3 days). Despite our best efforts, going to the gold store seems to remain the best way to get our hands on gold.
© 2020 Zs. Elter, P. Andersson and A. Al-Adili