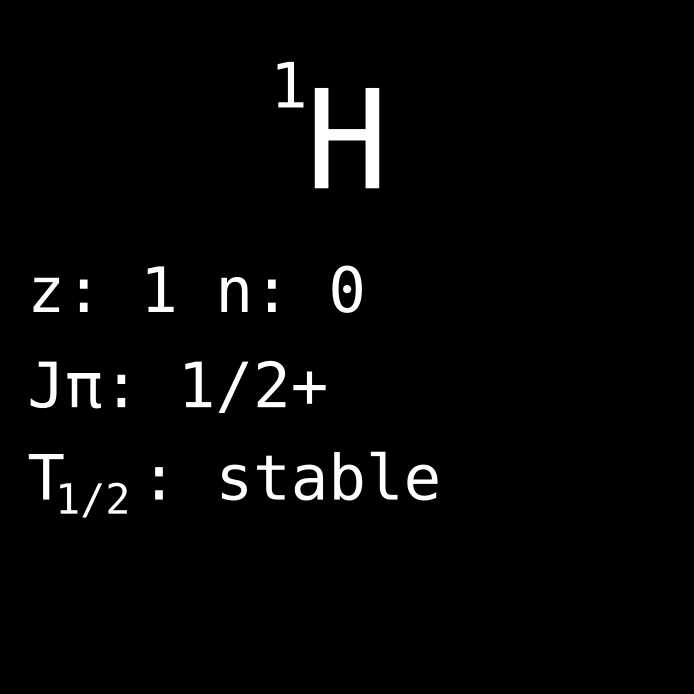

Light hydrogen (dog tag: H-1, nickname: protonium) is the simplest nuclide that can be found in nature in a stable configuration. The nucleus of hydrogen is simply a single proton, and the atom is just an additional electron flying around the nucleus. It is so elegant that Doctor Manhattan decided to mark his forehead with the atomic structure of hydrogen.
Light hydrogen was the first stable nucleus to form in the young cooling universe, and it still makes up about 90 % of the atoms in the universe. Once the light hydrogen was contracted by gravity to form the first stars, the gravitational contraction made it warm. The protons were like a giant pile of miniature billiard balls, bouncing around. But unlike the billiard balls, when hot enough, the hydrogen started to undergo thermonuclear fusion, producing deuterium, followed by helium, and subsequently all other nuclides that fill our universe, by nuclear reactions and radioactive decay. But it all started from light hydrogen.
Although in principle a neutron could be an as simple nucleus as hydrogen, it couldn’t be an atom, since it is neutral. It is also not stable, and in fact it happens to decay with a 15 minute half-life to a stable proton, thus hydrogen-1. The reason for this is that the proton has a slightly smaller mass.
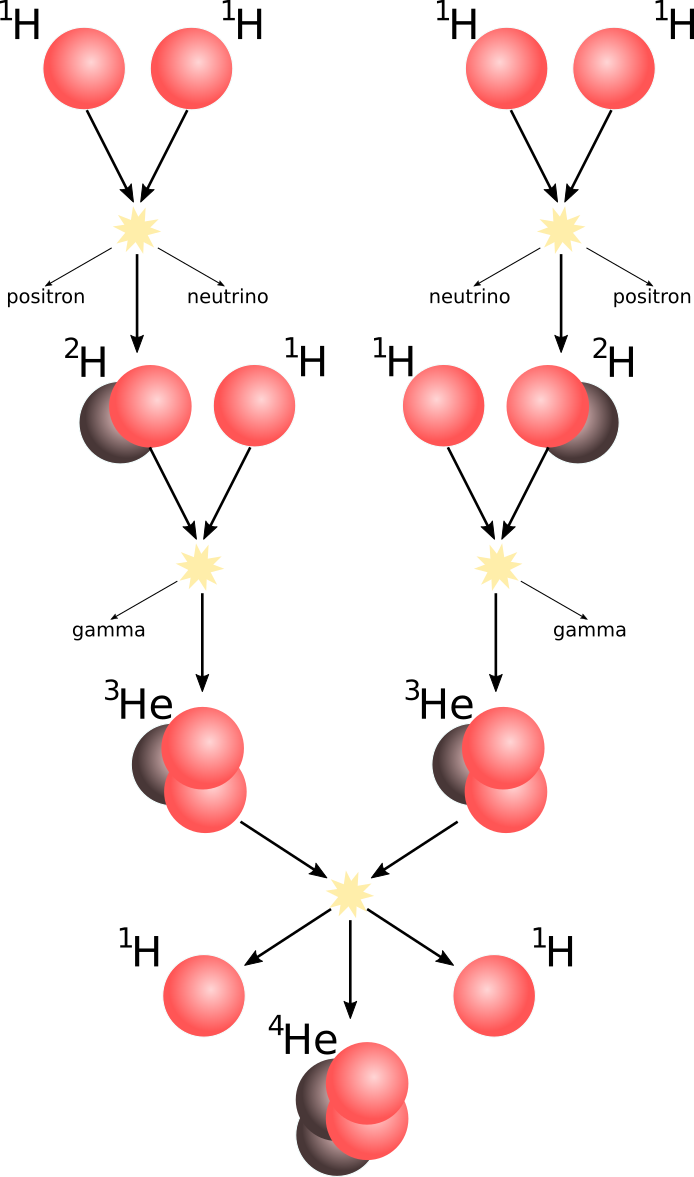
In our own sun, the hydrogen fusion reaction is responsible for the majority of the heat production. In that way, our lives and ecosystems depend on the nuclear energy potential between light hydrogen nuclei. The fusion of hydrogen-1 in the sun is a slow process, and therefore the sun can continue heating for billions of years. This is because the fusion of two H-1 results in helium-2 (He-2) consisting of two protons. This nucleus is not a bound system, and therefore needs to simultaneously experience a decay of one of the protons to a neutron, to form H-2. This “reversed” decay is possible only because of the higher binding energy of H-2 as compared to He-2.
© 2020 Zs. Elter, P. Andersson and A. Al-Adili